Do you have an open MCT4 backdoor, or you have a low back pain?
The name of this blog, MCT for health, is originally derived from the name of medium chain triglyceride, MCT oil. However, as I am gradually finding connections with lactate metabolism, it could easily be derived from MCT transporters, such a passable protein gates in cell membranes. I follow up on the previous post, which clarifies the behavior of fat cells and the causes of their dysfunction, which is manifested by a change in glucose metabolism and lactate production, thus triggering pseudohypoxia. So we explained that triggering these mechanisms in the fat cell is not desirable, but it is a rescue mechanism to deal with the extremely high insulin sensitivity caused by excessive polyunsaturated fats.
Today, however, we find out that the exact same mechanism in another tissue is completely fine and beneficial. We even find that it causes problems when pseudohypoxia doesn't start, when the gates of MCT4 transporters don't open. As you can see, nature has given us processes that are designed for specific situations and specific tissues. However, if they are triggered in a different situation or in a different tissue, they can cause us problems.
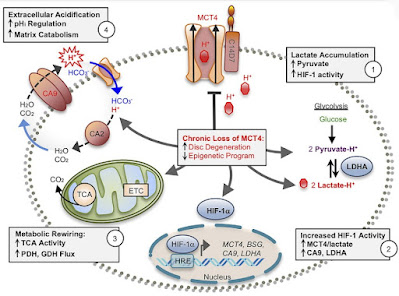
In this study, the authors investigated how the production and release of lactate affects the health of the spine and discs. That is, tissues that under normal conditions do not have sufficient access to oxygen and use glycolysis and fermentation of glucose to lactate as a standard process for obtaining energy, ATP molecules. And they came to some interesting findings. Degeneration of the intervertebral discs and low back pain are related to the overall health of the spine and vertebrae. The reduced bone volume and disrupted architecture in mice with knocked out MCT4 transporters clearly indicate that the release of lactate from cells through this backdoor is essential for maintaining bone quality. Recent studies have shown that osteoblasts preferentially use glycolysis for ATP production, even under aerobic conditions, thus mimicking the Warburg effect. Thus, it is possible that loss of MCT4 may directly affect the metabolic profile of osteoblasts and disrupt proper trabecular bone formation and metabolism in vertebrae.
The study further shows a link with the transcription factor HIF-1α, which controls the programming of cells for an oxygen-poor environment. We already know this from other studies. If we put all the findings into context, we can describe the mechanism by which the insufficient opening of MCT4 transporters can occur, and this leads us again to the problem of excessive consumption of polyunsaturated oils, especially omega-6 linoleic acid.
So how can this happen? Under normal circumstances, in these tissues, glucose is used to produce ATP through glycolysis with the production of lactate. This lowers the pH inside the cell (increases the acidity inside the cell) and increases the concentration of lactic acid, lactate. This, as we already know, induces pseudohypoxia, the expression of HIF-1α, the opening of MCT4 transporters, and the release of lactate out of the cell into the intercellular space and into the blood, allowing continued production of the necessary ATP molecules. Lactate is taken into the blood and processed, for example, in the liver. This requires a certain optimal concentration of hydrogen peroxide to allow HIF-1α to stabilize and activate the correct genes and continue this way of obtaining energy. However, excessive activity of glutathione peroxidase (GPx), which is caused by long free fatty acids, especially linoleic acid, must not occur. Impaired fat metabolism, increased free fatty acids, increased activity of glutathione peroxidase, decreased concentration of H2O2, insufficient stabilization of HIF-1α, insufficient activity of MCT4, excess lactate in the cell, lack of ATP and hyperacidification of the intercellular space, this is a sequence of problems that lead to metabolic reprogramming cells, which eventually manifest in the disruption of the structure of the vertebrae and discs, manifest in spinal pain and changes in the structure of the tissue.
A potential solution in the early stages could be to support a stronger response to hypoxia and increase HIF-1 expression, for example, with hydrogen peroxide or ozonated olive oil (at the end of the post).
Resources:
Lactate Efflux From Intervertebral Disc Cells Is Required for Maintenance of Spine Health




Comments
Post a Comment