What is cellular senescence? Could it be behind obesity, cardiovascular disease, or even Alzheimer's?
I will try to summarize the latest findings as I have come to them from published studies, which strongly support my original idea that metabolic problems occur when a certain number of cells switch from oxidative to fermentative metabolism in tissues or cells that do not normally do this. These can be fat cells, liver cells, or supporting cells of the nervous system, but also cells of the bone marrow, the immune system, etc. A very wide range. In this blog, I have so far called this phenomenon pseudo-hypoxia, i.e. a state where a cell activates the same mechanisms as in a lack of oxygen, but in a situation where there is enough oxygen, in so-called normoxia. This condition also manifests itself as chronic inflammation, so many researchers consider inflammation to be the cause, without knowing what the specific cause is.
From now on, I will call this state senescence. Why? Because senescence is the official term for a cell in a state where it has stopped its development, waiting and just surviving, but it does not obey the command to self-destruct, to apoptosis. It is probably waiting for better times (repair of slightly damaged DNA - added), but they usually do not come. What is very, very important, the senescent cell does not keep it to itself. It shouts this information out in all directions using chemical signals. It announces to the whole body and, therefore, to all cells: "Hello, I am here and I have a problem, can someone do something about it?" and so the other cells also willingly go into this state for preventive reasons and shout the same message, so the message is amplified and spread. But the signal is false, nothing fundamental is happening, but how to explain that to stressed cells, right?
 |
| Selective removal of senescent cells in adipose tissue corrects insulin resistance. |
How does the senescence of a few cells lead to obesity?
The primary factor in the development of obesity is changes that occur in adipose tissue, most likely around the internal organs, i.e. visceral fat. Only a small percentage of cells change. The change is caused by an overload of some metabolites, which would normally lead to higher fuel burning or storage, but suddenly, the cell hits a limit, and it can no longer do so. Why can it no longer do so? NADPH has run out. After all, more of it cannot be produced because the mitochondrial enzyme IDH2 or the cytosolic line for the production of NADPH, i.e. the PPP pathway, cannot be activated more. For this reason, the antioxidant chain cannot be activated more and this is manifested externally by higher production of hydrogen peroxide (H2O2). That is a signal. Normally, in such a situation, more fat is created through de novo lipogenesis (DNL), but it also needs NADPH. It does not work as it should. So the defense, senescence, is triggered. Just a few cells need to be switched on, and obesity is set in motion. Senescence is triggered via H2O2 by activating the PLA2 enzyme and separating parts of arachidonic omega-6 polyunsaturated fatty acids from the phospholipids of the cell membrane and oxidizing them into so-called oxylipins. These then activate the PPARγ factor, which triggers the state of senescence. It, therefore, depends on the amount of H2O2, the composition of the cell membrane, the current effectiveness of antioxidant protection, etc. It can be influenced a lot, for example by what we have eaten historically (the composition of our cell membranes) and what we eat now (what fats and antioxidants). But if a cell has already switched to senescence, it cannot be reversed, or it can only be reversed with difficulty. The best way to help is to remove senescent cells through apoptosis because there are not many of them. Senescent cells affect the entire organism, causing insulin resistance and metabolic syndrome.
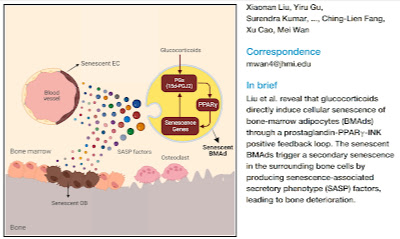 |
Glucocorticoids activate the senescence of fat cells in the bone marrow, which activates bone loss. |
How can we prevent cellular metabolism from becoming overloaded and, thus, cells from entering senescence?
There are certainly many possibilities. The main thing is to reduce NADPH consumption. This can be done, for example, by turning off de novo lipogenesis (via ACC phosphorylation), which can be done, for example, by acetate produced by intestinal bacteria, which is transferred by the liver to the entire bloodstream for use where it is needed.
Some researchers claim that acetate easily enters the cell through the membrane, but I don't think so. It probably requires activating the MCT1 membrane transporters, otherwise the acetate level would have to be too high. So for acetate to enter the cells, lactate must also be present in the blood, which activates MCT1. This ensures that acetate gets to the right place and in the right amount. Where there is smooth fuel burning and oxidative phosphorylation, there is a minimum of lactate, but where glucose is fermented, there is enough lactate. It is this that probably helps to let acetate into the cell and increase the level of cytosolic acetyl-CoA, which turns off pseudohypoxia (the HIF1A factor). This can restore oxidative phosphorylation. Acetate can do this in cooperation with lactate. Specifically, this means that, for example, exercising that increases lactate production will allow acetate to enter the cells better and improve metabolism. However, honey or sugar in small quantities will have a similar effect, for example.
In the long term, it is necessary to ensure the correct composition of cell membranes. This is mainly due to the composition of fats in the diet, it is mainly necessary to limit omega-6 fats, which are the raw material for the production of arachidonic acid and oxylipins. Omega-3 fats are better, both because they activate the production of acetate in the colon (ALA, linseed oil), and because they are a significantly better material in membranes (DHA, EPA) than omega-6 fats because, after their separation with H2O2, other substances are formed, significantly less toxic. In any case, it is better not to use polyunsaturated fats as a source of energy, but only in small quantities as a building material.
Antioxidants, such as vitamins C and E, can also help reduce H2O2 levels, although, unlike acetate, only as a preventative measure. They can reduce oxylipin production by reducing oxidative stress, but they cannot correct metabolism. Only short-chain fatty acids, especially acetate, can do this. For example, diluted vinegar (acetic acid) can probably be used as a dietary supplement in a concentration of up to 1% aqueous solution administered continuously throughout the day in small doses in a total amount of about 0.5 liter per day, experimentally.
How do atherosclerosis and vascular diseases arise?
How does bone thinning arise?
How does Alzheimer's disease probably arise?
Everything arises in exactly the same way. First, senescence occurs in some organs and it may not always be adipose tissue. Senescence spreads throughout the body via so-called SASP factors, chemical signals produced by senescent cells. In fact, senescence is the opposite of cancer. Stopped cell division and reduced cell renewal, unlike cancer, which is known for its rapid and unstoppable cell division. The accumulation of senescent cells may even cause cancer. For example, oxidative stress caused by turbulent blood flow causes senescence of the vascular endothelium, which, over time, leads to atherosclerosis and vascular diseases. Similarly, senescence of fat cells in the bone marrow leads to osteoporosis.
Senescence has been known for a long time, but it is not commonly known. Not everything has been researched, new works are published every day. But I think it is a very good clue and will lead to a real solution to the causes of many health problems. Moreover, we already know that senescence can probably be suppressed with acetate/vinegar. We will soon see if there are any studies on humans. So far, there are only a few studies, mostly on rodents, almost nothing on humans yet.
References:
Targeting p21Cip1 highly expressing cells in adipose tissue alleviates insulin resistance in obesity
Inhibiting HIF-1 signaling alleviates HTRA1-induced RPE senescence in retinal degeneration
The impact of cellular senescence in human adipose tissue
Oxylipin-PPARγ-initiated adipocyte senescence propagates secondary senescence in the bone marrow
Gut bacteria identified in colorectal cancer patients promote tumourigenesis via butyrate secretion



Comments
Post a Comment