Are there cancer-causing gut bacteria? Is butyrate carcinogenic?
In the last few posts, I have focused on whether the main substance produced during a ketogenic diet, β-hydroxybutyrate, could cause heart damage. The results are not clear, because the described mechanism should actually cause longevity. So something appears that is completely normal in nature, namely that SIRT7, which in principle has a very positive effect, can have a negative effect in some situations. But that is normal. The amount determines whether a substance will be a medicine or a poison, right? The same probably applies to butyrate, a short-chain fatty acid with four carbons, produced by intestinal bacteria. It seems that it can also be dangerous, it can cause colon cancer via cellular aging, senescence.
 |
| Genetic removal of the ability of bacteria to produce butyrate (Mutant strain) leads to a reduction in the size and number of colorectal tumors in a mouse model of cancer. |
I found a study called "Gut bacteria identified in colorectal cancer patients promote tumourigenesis via butyrate secretion". The study seems to be really well-conducted, with many experiments demonstrating the direct influence of certain bacteria on the development of colon cancer. Bacteria that differ from others in that they produce butyrate in addition to acetate. And the main role in this is played by the "senility" of cells, sorry, senescence. The authors first identified 12 different strains of bacteria that are overgrown in the intestines of colorectal cancer patients.
 |
| Intestinal bacteria produce short-chain fatty acids, the indicated strains produce significantly more butyrate than other strains. |
Then they tested these 12 strains in a mouse model and identified two strains that caused problems. All the strains of the studied bacteria produce mostly acetate and propionate, but both of the identified carcinogenic strains also produce significant amounts of butyrate. So researchers began to investigate whether butyrate could be the cause of the carcinogenicity of these bacteria. And the results really lead to the conclusion that butyrate activates the senescence of intestinal epithelial cells. This senescence develops into a tumor over time. They even genetically modified the bacteria in such a way that they damaged the enzymes involved in the production of butyrate, and the carcinogenicity of the bacteria disappeared.
 |
| Butyrate-producing bacterial strains (red) induce senescence in intestinal epithelial cells, arresting their development. This subsequently leads to cancer. |
Each short-chain fatty acid (acetate, propionate, butyrate) seems to act differently. For example, another study shows that the liver tries to prevent butyrate from entering the bloodstream from the colon. It even prevents propionate from entering the systemic bloodstream. On the other hand, acetate produced by gut bacteria is readily released into the entire bloodstream. Acetate is simply the best, we've known that for a long time, right?
 |
| Acetate, not butyrate or propionate, is released from the colon throughout the body to do what needs to be done. |
But let's also focus on senescence, i.e. the aging of cells. In the literature, we learn that an old, poorly functioning cell can undergo apoptosis, i.e. break down into its components. It can do this on its own or someone else's initiative, and the main signal for apoptosis is hydrogen peroxide H2O2. However, instead of apoptosis, it can often fall into senescence. Basically, it suppresses metabolism, does not divide, interferes, but does not eliminate itself at the request of the immune system, i.e. at the command of H2O2. Over time, such a cell can develop into a tumor germ, probably due to a mutation of a gene caused by an already disturbed metabolism. |
 |
| Increased production of ROS (mainly H2O2) is a manifestation of chronic inflammation (NOX2 activation). |
You already know from previous posts that I am very interested in the phenomenon of (pseudo)hypoxia, i.e. the transcription factor HIF1A (hypoxia inducible factor 1 alpha). It is usually activated by oxygen deficiency, but it can also be activated by a stopped TCA cycle or a stopped electron transport chain (ETC) in mitochondria, i.e. by slowed oxidative metabolism (OXPHOS). An internet search engine usually pulls up interesting studies when a HIF1A factor is involved in a query. And senescence is no exception.
I came across many studies that confirmed my assumption that senescence is actually a manifestation of pseudohypoxia, i.e. the activated transcription factor HIF1A. Genetic or pharmacological shutdown of the HIF1A factor prevents cell senescence.
 |
| Senescence of retinal pigment epithelium and photoreceptors induced by HTRA1 activation can be suppressed by inactivating the transcription factor HIF1A by KC7F2. |
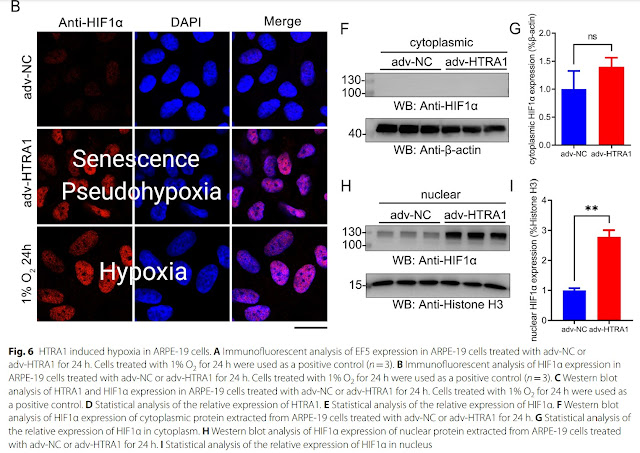 |
| Senescence induced by HTRA1 activation activates the transcription factor HIF1A, as does true hypoxia (1% O2). |
We already know that one of the important factors that helps activate HIF1A are vegetable oils containing too much omega-6 polyunsaturated fatty acids (LA) and too little omega-3 (ALA). These should be avoided. They cause acetate deficiency, destroy antioxidant protection through high consumption of NADPH and apparently also damage the gut microbiome by producing more butyrate and less acetate. Omega-3 (ALA) is an important substrate for bacteria that produce acetate.
If we cannot rely on bacteria to produce enough acetate for us, it would probably be appropriate to supplement acetate from the diet, supplementing it continuously 24/7 (about every 2 hours) in small doses in the form of diluted vinegar (about 0.5 liter of 1% vinegar solution per day), because high doses can have the same negative effects as alcohol consumption. Vinegar is able to acetylate HIF1A and ensure its removal, i.e. deactivation of pseudohypoxia. It should therefore also support the removal of senescent cells, whose metabolism has been shifted by the activated HIF1A factor so that they cannot be removed. Whether this really works in humans is a question, as it is only a hypothesis based on findings from studies on animal models.
Addition
Acetate does indeed appear to have an anti-senescence effect, as this study shows.
References:
Gut bacteria identified in colorectal cancer patients promote tumourigenesis via butyrate secretion
Role of hypoxia in cellular senescence
Hypoxia-Inducible Factor-1α: The Master Regulator of Endothelial Cell Senescence in Vascular Aging
Hypoxic State of Cells and Immunosenescence: A Focus on the Role of the HIF Signaling Pathway
Inhibiting HIF-1 signaling alleviates HTRA1-induced RPE senescence in retinal degeneration
The impact of cellular senescence in human adipose tissue




Comments
Post a Comment