The mystery of the missing Acetyl-CoA molecule, acetate as a solution?
What is cytosolic acetyl-CoA? We all know vinegar, it is an acid containing two carbons, the shortest fatty acid. We have already met MCT oils here, which contain medium chain fatty acids with eight or ten carbons, coconut oil contains lauric acid with twelve carbons. So if we go in the direction of fewer carbons, we get to butyrate in butter, a fatty acid with four carbons, and acetic acid with two carbons. If the acid is dissolved in water, hydrogen is separated as a proton and we are talking more often about the molecule of acetate, butyrate, oleate, etc., not about the acid as such. That's just an introduction.
But what is acetyl-CoA? Acetate is a small molecule, so that the cell can control the flow of these small molecules, it attaches a part called Coenzyme A abbreviated CoA to them. The process of attaching acetate to CoA requires energy (ATP) and it's actually the activation of the acetate molecule for use in the cell. This process sends out a signal (AMP) to support energy production (activates AMPK). But this process only takes place in the presence of acetate. If acetate is not available, another, more complicated pathway is used.
Acetyl-CoA molecules are common, they are produced in the process of metabolizing carbohydrates, proteins and fats. But this takes place inside the mitochondria, the cell organelles for ATP production. If ATP is made in the cell plasma, i.e. the cytosol, no acetyl-CoA is produced. There are only two ways for acetyl-CoA to enter the cytosol. I already mentioned the first route, from acetate by the enzyme ACSS2. The second route is from citrate released from mitochondria processed by the enzyme ACLY. The necessary condition is therefore a functioning metabolism in the mitochondria (TCA cycle), an excess of citrate such that it is released outside, and it is also conditioned by a sufficient amount of cytosolic malate, which is used for the exchange with the mitochondria for citrate. So it's quite complicated. Additionally, if ACLY produces acetyl-CoA, it can be taken up by either for the formation of new fat (ACC) or for the pathway of NADPH production by isocitrate dehydrogenase from citrate. So even if enough of citrate is produced, it may be missing due to over consumption. But why am I actually addressing the availability of cytosolic acetyl-CoA?
I found one very interesting study when I was trying to find the mechanism by which short fatty acids have such a fundametally positive effect on metabolism. Let us briefly review here what adding acetate to the diet of mice can do. It is enough to add 5 percent by weight of sodium acetate to the diet of mice so that they do not gain weight at all on a fatty diet! In addition, we can even make them fat without acetate and then add acetate to their diet and the mice will lose weight on their own, without any calorie restriction, they can eat as much as they want. Big mystery, right?
 |
| PPAR-gamma is controlled by the transcription factor HIF-1α. |
So when I was looking for a possible mechanism of how this is possible, I found a study that shows how the transcription factor HIF-1α, i.e. oxygen deficiency signaling, can be activated even in a situation where there is enough oxygen, the so-called normoxia. I have already shown here how the activation of HIF-1α controls the so-called hypertrophy of fat cells, their enlargement. Just genetically turn off HIF-1α and the fat cells stay small. It is the diseased large fat cells that cause changes in the entire organism, sending out signals that cause damage to other organs, especially the liver, but can also support the development of cancer.
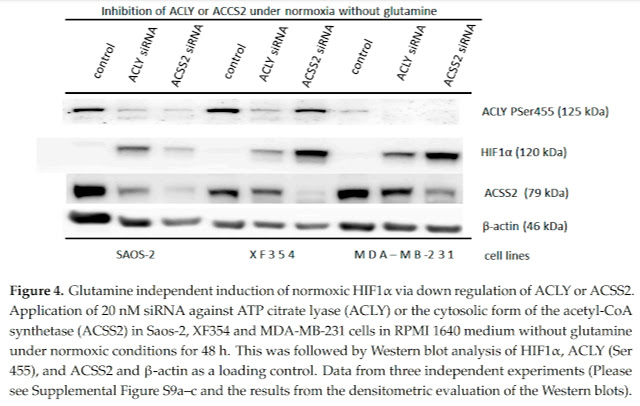
The study shows us that in normoxia it is important to maintain enough cytosolic acetyl-CoA and thus the functioning acetylation of molecules, in this case the acetylation of HIF-1α molecules. Thus, they are marked for destruction and are thus removed. So it's the same as genetically turning off HIF-1α. And in the study, the authors show us that acetyl-CoA deficiency can be induced by adding glutamine, an amino acid that, when processed, releases ammonia, which changes the pH in the cytosol, and the activation of HIF-1α allows pH to be equalized by releasing lactate and converting CO2 to bicarbonate.
I therefore believe that the positive effect of acetate, which naturally arises as a product of intestinal bacteria, as a product of fat metabolism in peroxisomes, or as a product of alcohol processing in the liver, is due to the ability to acetylate the transcription factor HIF-1α and thus erase the signal of pseudohypoxia in adipose tissue. In addition, acetylation is required for the proper functioning of the control of gene activation in the cell nucleus. Thus, maintaining functional acetylation is essential for health and for the functional metabolism of all body organs.
 |
| One more picture of how short fatty acids subsequently affect obese mice in such a way that they lose weight in 6 weeks. |
Supplement
Acetylation of HIF-1α, on which deactivation of pseudohypoxia depends, may not be the only protein controlling metabolism by acetylation. A number of studies prove that the activation (phosphorylation) of AMPK is not only controlled by a lack of ATP and an excess of AMP, but by the presence of glucose itself, i.e. the presence of a product of glycolysis called fructose-1,6-bisphosphate (FBP), which binds to the aldolase enzyme and is functioning as glucose sensor to control AMPK. And it is the control of aldolase and glycerol-3-phosphate dehyddrogenase activity by acetylation that can play an essential role in the positive effect of acetate on metabolism. So far, two studies can serve as proof of the statement. The first shows how controlling the aldolase enzyme changes metabolism, and the second shows how acetylation of aldolase changes aldolase activity by about three orders of magnitude.
 |
| The aldolase blocker aldometanib corrects the metabolism of mice on a high-fat diet. |
 |
| Acetylation blocks the activity of aldolase. |
References:
Causes and Consequences of A Glutamine Induced Normoxic HIF1 Activity for the Tumor Metabolism
Production of acetate in the liver and its utilization in peripheral tissues
Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK
The aldolase inhibitor aldometanib mimics glucose starvation to activate lysosomal AMPK









Comments
Post a Comment