Omega fat oxidation as a prevention of civilization diseases?
Omega oxidation of fat, what is it? You've probably never heard of this term before, in fact I think most professionals either don't know the term or have long since dismissed it as irrelevant information. I will try to explain here why I think that omega oxidation, or the insufficient activation of this metabolic process, is the root cause of civilizational diseases, including cancer.
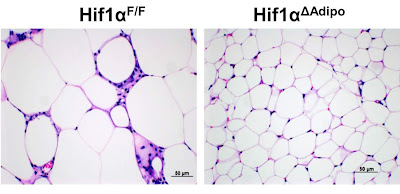
But let's start slowly. In one of the previous posts, I explained the activity of the fat cell. As long as it is not overloaded with excess fuel, free fatty acids (FFA / NEFA) or glucose, the cell works normally. If there is more fuel, it is stored as fat, if there is not enough, it releases fat into the bloodstream. Very useful activity. But studies on mice show us that if we give them a high-fat diet instead of a standard chow diet, the mice will get fat and become obese. Their fat cells will grow in size (left image), stop working properly, start to signal inflammation and releasing excess fuel in the form of lactate.
This is a known fact that ultimately led to bad recommendations. I already explain in this post what is wrong with it. But let's move on, the release of excess fuel in the form of lactate means that a state of pseudohypoxia has been activated, the transcription factor HIF-1α has been activated, so increased fat formation and storage has also been activated. This explains the excessive enlargement of fat cells. What triggered HIF-1α if it wasn't lack of oxygen? What triggered its stabilization? How is it that HIF-1α was not tagged with the -OH hydroxyl group by the PHD enzyme, prolyl hydroxylase? What affects the activity of the PHD enzyme? It turns out that PHD activity can be stopped by accumulating a by-product of PHD activity, the already well-known succinic acid, or succinate, an intermediate of the TCA (Krebs, citrate) cycle.
A normally functioning cell is able to easily remove the byproduct of hydroxylation, succinate, using the SDH enzyme, succinate dehydrogenase, mitochondrial complex II. Thus, any problem with the activity of the second mitochondrial complex increases the likelihood of triggering pseudohypoxia. We also already know that slowing down or stopping can be induced with malonate, a false substrate that binds to the SDH entry site instead of succinate and disables its function. Another substance that can potentially stop the activity is an excess of the resulting byproduct, which is FADH2. Perhaps this whole chemical reaction can be inverted using an excess of FADH2. How does this all relate to fat metabolism? Quite fundamentally.
But before we explain it, let's look at one more study that Georgi Dinkov recently commented on his blog. It shows, similar to my previous post, that in certain situations the cell uses shortcuts. Processes that normally take place inside the mitochondrial matrix are replaced by processes taking place in the cytosol, the cell plasma.
Thus, metabolic cycles similar to the TCA cycle can be found, but which do not need oxygen, nor do they produce lactate. On the other hand, they break down and create fats, while using energy from glycolysis. A number of TCA cycle processes are stopped or even run in the opposite direction. Thus, TCA cycle intermediates can be moved out and used to convert NADH to NAD+ instead of fermenting pyruvate to lactate to ensure the continuous breakdown of glucose and thus provide ATP energy. What these processes have in common is that they do not use the second mitochondrial complex, SDH. Thus, they activate pseudohypoxia and are further maintained by it because HIF-1 activates fat production, which keeps this cycle going. The same condition also shuts down the system for regulating the entry of long fatty acids into the mitochondrion, because it is also dependent on the activity of PHD, so these are processes that are limited only by the availability of fuel and that is very, very dangerous.
Thus, if the processes described in the previous paragraph are activated, the beta oxidation of fats takes place at full speed, but the resulting intermediates will never be oxidized and released in the form of CO2 and water, but will be stored as new fat. At the same time, many FADH2 molecules will be formed, they will probably turn the direction of SDH to the formation of succinate from malate, so the activation of HIF-1 is permanent and it will not be easy to restore the original healthy processes. Also, many NADH molecules will be produced, which will reverse the direction of part of the TCA cycle so that citrate is produced from α-ketoglutarate. And citrate is normally the main brake on excessive fuel intake, but it is also a substrate for the formation of fats, so it is intensively taken up and therefore cannot act as a brake. This is the metabolism of cancer, this is how the authors of the aforementioned study see it.
We left the fat cells of mice that gained weight erratically on a high-fat diet. But the authors of the study genetically modified a group of mice in such a way that they disabled HIF-1 selectively only in fat cells. What happened? The high fat diet stopped bothering the mice, no obesity, no insulin resistance or blood sugar problems, just nothing (right image of fat cells). The high-fat diet was suddenly completely healthy for the mice thus modified. How to explain it?
You may have already guessed. If fats slow down or stop the activity of SDH, the second mitochondrial complex, activation of HIF-1 through the accumulation of succinate will take care of the rest. If HIF-1 stays turned off like in engineered mice, nothing bad happens. And what about a human? Want to turn off HIF-1? I wouldn't advise you to do that. We don't have to genetically turn off HIF-1 in adipose tissue. After all, it is enough to ensure sufficient SDH enzyme activity. Nothing more is needed, and we already know how to do it. It is sufficient to ensure enough of substrate for the activation of SDH, i.e. succinate. In addition, succinate is not only a substrate, but apparently controls many processes through the receptor. What is certain is that succinate acts as an agent to arrest pseudohypoxia and to restore the normal response to true oxygen deprivation. We have already shown this with the example of a heart attack.
Now let's go back to omega oxidation. Fatty acids are made up of carbon chains that have a carboxyl group -COOH at one end. They can be metabolized in mitochondria or in peroxisomes. Long, medium and short are processed in the mitochondria. Peroxisomes mainly process long and very long fatty acids (and they even prefer to process unsaturated and polyunsaturated and also dicarboxylic). The final product of beta oxidation is a molecule of acetyl-CoA and the appropriate number of molecules of NADH and FADH2 (or NADH->malate/lactate and FADH2->H2O2 in peroxisomes), no ATP. Some of the fatty acids, especially of medium length, are modified by omega oxidation even before entering the mitochondrion, and the other end is then terminated with a carboxyl group -COOH, they are called dicarboxylic acids and are thus accurately transported to peroxisomes. The resulting product of beta oxidation is not acetyl-CoA, but acetate, succinate and succinyl-CoA, which enters the TCA cycle on the opposite side and ensures the activity of SDH. Keeping SDH active is vitally important in the metabolism of fats, so it is necessary to always maintain an appropriate ratio of medium and short chains of fatty acids to long chains. Medium chains activate omega oxidation, even of long fatty acids. If they had used some medium-chain fats in the study on genetically engineered mice, they probably wouldn't have had to genetically engineer the mice. The future will show whether this is really the case, perhaps such studies will be carried out. The preventive effect of MCTs against metabolic problems has already been proven in studies, but no one has yet clarified why it works. Additionally, we know that omega oxidation increases during fasting. Evidently, the body itself knows what to do to burn fat, it needs omega oxidation. Also common acetylsalicylic acid (Aspirin) increases omega oxidation, which is perhaps its most important, although practically unknown, effect. Even such that Aspirin significantly reduces the risk of cancer.
Supplement:
Omega oxidation of saturated fats increases the production of acetate by burning dicarboxylic fatty acids in peroxisomes, thus activating the enzyme AMPK and blocking the synthesis of fats and promoting their burning in the mitochondria. This is probably even more important than the production of succinate.
References:
A “Weird” Mitochondrial Fatty Acid Oxidation as a Metabolic “Secret” of Cancer
Contribution of omega-oxidation to fatty acid oxidation by liver of rat and monkey
PHD3 Loss in Cancer Enables Metabolic Reliance on Fatty Acid Oxidation via Deactivation of ACC2
Induction of Omega-Oxidation of Monocarboxylic Acids in Rats by Acetylsalicylic Acid
A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence
Supplement of Succinate Reduces Lipid Deposition and Improves Metabolic Function in Obese Mice





Comments
Post a Comment