From pyruvate to lactate or from lactate to pyruvate? That is the Question!
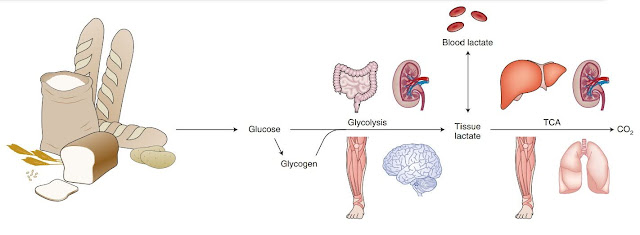
Lactic acid, or lactate (not to be confused with lactose - milk sugar) is such an ugly duckling, pushed aside. It is considered by most to be an insignificant product of tired muscles that tells us when we have had enough and that we should rest. However, this is a very narrow view. I have already mentioned here that we have an immediate supply of glucose in the blood equivalent to only about one teaspoon of sugar. It is said that we have up to 20 times more lactate in our blood! Well, I don't have proof of that, but it's certainly a fuel that needs to be reckoned with, not ignored. Every cell can convert lactate to pyruvate and vice versa. Don't you know what pyruvate is? This is a product of glucose processing in the cell. Once the cell takes in glucose, it tags it with a phosphate tag and the glucose can no longer escape from the cell. There is no going back for glucose. It will be converted to pyruvate. The fate of glucose in the cell is determined by many things. If we take a muscle cell, for example, glucose can be stored in the form of glycogen for later use. Or, in the presence of oxygen, it can be used to produce chemical energy, namely the ATP, while water and CO2 molecules are also formed. Or if there is not enough oxygen, it can be used to produce ATP energy without the need for oxygen by converting it to lactate and as lactate it can also leave the cell. Or it can be processed differently according to the current need, e.g. converted to fat.
Who and why decides the fate of glucose molecules in the cell? Above all, needs and possibilities decide. In general, an increasing level of the product of a chemical reaction gradually slows down and stops the reaction. Since practically all chemical reactions in the cell are mediated by enzymes or rather enzymatic pathways (complex production lines), the quantity and quality of these nanomachines as well as the availability of so-called cofactors, substances required for the realization of a given reaction, decide which reactions will prevail and which will be suppressed. Signals from the surrounding environment influence the so-called expression of genes (i.e. the availability of production plans and procedures) for the preparation of individual enzymes (nanomachines).
An example of lactate as a signaling molecule for communication between cancer cells.
Now let's get back to lactate, which is a great vehicle for intercellular exchange of fuel. We have many different cells in the body, not all of them have ideal conditions, not all of them have the necessary enzymes, not all of them have enough oxygen. So obtaining energy through oxidative phosphorylation (combustion with the help of oxygen) is unavailable for many cells. They have no choice, they have to process glucose and convert the resulting pyruvate into lactate and release it back into the bloodstream to be processed by other cells (by the liver, kidneys or heart) that have everything necessary for oxidation (the so-called Cori cycle ). They take lactate and convert it back to pyruvate. Then they convert it to Acetyl-CoA and send it to the Krebs (TCA, citrate) cycle, which produces NADH and CO2. The NADH is sent to the electron transport chain (ETC), which, like in a power plant using a turbogenerator (complex V), produces ATP and creates water from oxygen.
If there is not enough oxygen, NADH builds up as excess fuel. This stops the Krebs cycle, which will lack NAD+, leaving Acetyl-CoA in excess. This stops the conversion of pyruvate to Acetyl-CoA, so pyruvate also starts to be bought and the production of the enzyme LDH (lactate dehydrogenase) is activated, which ensures the conversion of pyruvate to lactate. And now it gets interesting. This release from the cell takes place via the membrane transporters MCT1 to MCT4. Attention, MCT here has nothing to do with MCT oil, but stands for monocarboxylate transporter, so it has nothing to do with the name of this blog. But finally we get to the crux of the lactate problem. The activity of the transport enzymes MCT1 and MCT4 is absolutely essential, MCT1 is used for transport both into the cell and out of the cell, MCT4 only releases lactate out of the cell. Not all cells have activated MCT4, and since MCT1 transports in both directions, its regulation by one need can cause a problem elsewhere. MCT4 have cells in which lactate production is not usual, MCT1 uses, for example, red blood cells, which normally produce lactate, for discharge. They do not have mitochondria and are therefore unable to oxidize glucose or fats. But what about fat cells? Do you think they don't produce lactate? They produce, especially when they have problems. And interestingly, lactate stabilizes the already well-known transcription factor HIF-1α, which signals and programs the cell as if it were in an oxygen-deficient environment, hypoxia. Thus, lactate turns on pseudohypoxia, as we described in previous posts.
What problems can fat cells have? They simply store fats in the form of triglycerides, take free fatty acids and attach them to one of the three bonds of glycerol. The resulting fat droplets serve as a perfect supply of fuel for the winter. It is interesting that in the fat cell there are constant processes of breakdown of triglycerides into free fatty acids and glycerol, and in addition, opposite processes of triglyceride formation from free fatty acids and glycerol also take place. This apparently makes it possible to respond flexibly to the needs of individual organs, to supply specific types of fatty acids that are currently needed. But the most interesting thing is that glycerol is not recycled, but the old one leaves the cell and the new one has to be made from glucose. New saturated and monounsaturated fatty acids are also formed from glucose so that the resulting fat consistency is just right, neither too runny nor too solid. So fat cells take in a lot of glucose. In fact, the formation and storage of fat in adipose tissues is directly controlled by insulin and the amount of incoming glucose. So they can also have insulin resistance.
Healthy adipose tissue contains small fat cells that do not prevent the storage of additional fat. But it is found that the adipose tissue containing large fat cells no longer wants to receive more fat and fights back, does not listen to insulin signaling and does not let glucose in, it has insulin resistance. How come the cells did not divide in time while they were still small and functional? Why did they get pumped up with fat to the point of bursting until they stopped working properly? I have an explanation, because insulin resistance is not always bad. On the contrary, it is necessary to protect against overfilling the cell with excess fuel. So what kind of food reduces insulin resistance in an unhealthy way? Well? After all, linoleic acid omega-6, if it occurs as a free fatty acid in the blood, if there is too much of it. Fat cells are damaged by their too high sensitivity to insulin, which is caused by vegetable oils from sunflower seeds, rapeseed, etc.
And how does this relate to lactate? The overfueled cell begins to get rid of glucose by converting pyruvate to lactate. Normally, the cell converts lactate to pyruvate in the presence of oxygen, but excess pyruvate causes this bidirectional reaction to reverse. So a lot of lactate is produced, but do we have enough MCT1 transporters in this situation? Well, we don't, because the overfueled cell resists further inflow of more fuel (lactate) from the surroundings and suppresses the MCT1 transporters. What about MCT4? Yes, lactate helps stabilize HIF-1α and through it also turns on MCT4. However, HIF-1α also turns on many other processes (e.g. fat synthesis), it turns on pseudohypoxia. So there you have it, pseudohypoxia, the Warburg effect also related to cancer, is a direct consequence of excessive insulin sensitivity of fat cells. It starts as excessive fat storage, originally planned by nature to get through the winter season, and continues with excess lactate and rescue metabolic attempts to cope with excess fuel in the cell, which may not always end successfully.
References:
Lactate: the ugly duckling of energy metabolism
Monocarboxylate transporters in cancer
Role of lactate transporter (MCT1) in skeletal muscles
Lactate stimulates CA IX expression in normoxic cancer cells
Lactate release from the subcutaneous tissue in lean and obese men.





Comments
Post a Comment