How does omega-6 vegetable oil confuse our cells?
It will probably be a little less digestible today, but I need to sort out my thoughts. It is time to make a small inventory of how specifically polyunsaturated vegetable oils, especially linoleic omega-6, affect cellular signaling in obtaining energy from available fuels and thus confuse the cell so much that it begins to behave completely differently than in the burning of conventional animal fats.
Let us now repeat what we already know clearly about linoleic acid:
- It devastates our reserves of reduced glutathione (GSH).
- Low levels of GSH strongly suppress the activity of the first mitochondrial complex of the electron transport chain, thus suppressing the production of energy from NADH molecules.
- Initially, it improves insulin resistance, thus reducing H2O2 levels.
- It reduces the activity of the enzyme superoxide dismutase (SOD).
- It supports the beta-oxidation of unsaturated fats, the desaturation of saturated fats, ie the conversion of saturated fats to monounsaturated, the cleavage of free fatty acid chains to acetyl-CoA, which is the fuel for the Krebs cycle, the output of which is mainly NADH. NADH and FADH2 molecules are also beta-oxidation products. Thus, after passing the acetyl-CoA molecules through the Krebs cycle, we have fuel for the first (NADH) and second (FADH2) mitochondrial complex. Little less FADH2, but it doesn't matter as I can show you.
And here we run into a problem, what about NADH? The first mitochondrial complex, the main consumer, is blocked due to low GSH levels. I propose to transfer NADH out of the mitochondrial matrix and use a glycerol-3-phosphate shuttle which processes NADH on mitochondrial complex III. However, this produces superoxide, but we obtain energy that is otherwise unavailable. We process FADH2 molecules in a standard way using complex II, this also produce superoxide. We get really a lot of superoxide as level of GSH goes down. Before this happens, extremely low H2O2 level (why we'll see later) cause extremely high insulin sensitivity and high glucose intake. This will charge mitochondrial membrane potential and generate also very much of superoxide. After the GSH reserves run out also the H2O2 level will increase and insulin resistance appear as the exact opposite of the previous state of extreme insulin sensitivity. (update: a higher level of H2O2 lets in more glucose at first, causing high sensitivity to insulin, but after gradual acetylation of enzymes, oxidation slows down and conversion to lactate starts, this triggers psehohypoxia)
Here we see that listening to foreign advice does not pay off, because I previously claimed that PUFAs produce less superoxide than saturated fats, influenced by it. This is not true, they, when burned, produce much more superoxide than saturated or monounsaturated fats, all studies say it. So we have a lot of superoxide, that slows down the Krebs cycle right at its beginning, by controlling an enzyme called aconitase. This is probably due to the fact that fat burning always produces a little superoxide to slow down the Krebs cycle, thus saving a little NAD+ for the beta-oxidation of fats. Superoxide levels can normally control the balance between fuels, fat and glucose. Superoxide degradation is carried out by the enzyme superoxide dismutase (SOD), which converts it to hydrogen peroxide (H2O2).
But we also already know that PUFAs reduce SOD activity, so it will only work slowly, at high levels of superoxide, so the Krebs cycle will be blocked. As a result, we will have an excess of carbon in the form of acetyl-CoA molecules. What about them? Well, we use them to make new fatty acids, new fats. We will even use acetyl-CoA carbon derived from carbohydrates, glucose. PUFAs are obeseogenic, this is known and confirmed by a number of studies, you simply get fat after them.
But why doesn't the peroxide level rise? This would prevent an excess of acetyl-CoA molecules in the cell and the cell would not "overload" with fuel. To clarify this, we need to look at another study. Linoleic acid has a special ability to tremendously activate the enzyme glutathione peroxidase. It converts H2O2 peroxide into water.
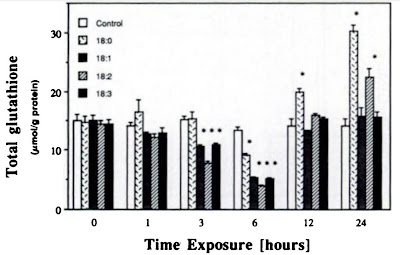
Linoleic acid can be seen on the graph as an open bar 18:2 versus an 18:0, which is a saturated stearic acid that is found in beef fat or butter, for example. In addition, you can see in the upper graph how this strong reaction to linoleic acid takes place over time. If the formation of new glutathione is still not impaired, it will be replenished, and the level may even increase. However, this will no longer be possible after the depletion of raw materials for production. In the short term, therefore, it will not cause any major damage, but in the long term it will.
This study, together with another explanatory study, actually forms such a hitherto little-known, missing connection to understand the whole problem caused by vegetable oils. Free fatty acids (non-esterified) strongly affect, and only by their presence, the activity of glutathione peroxidase (GPx), ie the rate of H2O2 elimination, via the epidermal growth factor receptor (EGFR). In addition, there are many interrelationships, eg EGF receptors are stimulated by hydrogen peroxide, support cell growth and division, and are therefore related to cancer. But surprisingly, although their numbers increase in the presence of EPA and DHA (omega-3 of animal origin), it slows tumor growth. So it's all very complicated and unexplored.
Until glutathione is consumed and its level is somehow restored, everything is seemingly fine. It just recharges energy storage for worse times (torpor). But glutathione diminishes over time, it is no longer able to recover, while the need does not decrease. What happens then? Glutathione peroxidase will be very active, but without glutathione it will not be able to remove H2O2. This causes an increase in its level and our well known insulin resistance occurs, an increase in insulin, then glucose, then the ability of the pancreas to generate such high levels of insulin, and we have the type two diabetes. All you have to do is continue in this state long enough.
One more interesting thing. With a local lack of oxygen, the cell must properly switch to low oxygen metabolism, fermentation. This is done by activating HIF-1, which ensures that the correct genes are turned on and off. It consists of two components, one of which, HIF-1a, is dependent on oxygen. In the presence of oxygen, it is marked for a degradation and is cleaned. Degradation must not occur if there is a lack of oxygen. It uses the fact that the state of oxygen deficiency (hypoxia) is usually accompanied by increased production of H2O2, which inactivates the degrading enzyme. Superoxide production seems to have no influence. There have long been disputes over what factors drive this transition and they are still going on. We will assume that the study is correct. What does this mean for us? Excessive production of hydrogen peroxide can potentially cause pseudohypoxia, a state in which HIF-1α is no longer degraded and oxygen is not used, even if there is enough. Fats will not be burned and instead energy will be obtained by fermentation, local acidification with lactic acid would occur.
However, the opposite problem can also occur, espetially in tissues poorly supplied with oxygen (bones, tendons, joints, etc.). If glutathione peroxidase is strongly activated, very low peroxide levels do not turn off degradation of HIF-1α, the cell does not express HIF-1 and could not resist hypoxia. This we see in the paper and this can cause cell death and inflammation. Without the presence of vegetable oils from seeds, this would not happen. I have already written about this in a post about chronic inflammation.
Once we are quite clear about the causes, we can afford to suggest a solution. Of course, prevention is ideal, do not eat vegetable oils with any omega-6 content. Neither cold nor fried. From birth, all life. What? You won't make it? You've already eaten tons of it? We will see what we can do.
Theoretically suitable remedy:
Restore glutathione (GSH) levels
- supplement glycine and ascorbic acid (vitamin C). This will allow glutathione peroxidase to work. It also awakens mitochondrial complex I and reduces superoxide production. It will also deplete H2O2 and increase insulin sensitivity, but can negatively influence sensing of hypoxia.
Reduce superoxide levels and speed up the Krebs cycle
- reduce blood sugar levels, ie reduce the amount of carbohydrates in the diet. Eat only shorter and saturated fats, that is butter, coconut oil, add MCT oil. Already in this older post, I showed you how MCT oil reduces the level of long free fatty acids in the blood.
Restore correct hypoxia behaviour
- if we have tissues poorly supplied with oxygen, with chronic inflammation, peroxide supplementation can help to respond them correctly to hypoxia. But I would also increase ascorbic acid intake to increase GSH to improve the chances of resolving inflammation.
Supplement:
How omega-6 oil disables the mitochondrial antioxidant system
References:
Fatty acid-mediated effects on the glutathione redox cycle in cultured endothelial cells
Fatty acids as modulators of the cellular production of reactive oxygen species
Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide
Glutathione Depletion in PC12 Results in Selective Inhibition of Mitochondrial Complex I Activity
Essential Fatty Acids Alter the Activity of Manganese-Superoxide Dismutase in Rat Heart
Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation





Comments
Post a Comment