Omega-6 polyunsaturated fats disable the antioxidant system of the mitochondria and thus increase the level of hydrogen peroxide in the cell!
I think the mechanism by which polyunsaturated fats increase the burden of oxidative stress in the cell has not yet been fully understood. Many people who recognize their toxicity are convinced that unoxidized oils are safe and non-toxic. However, scientific studies tell us that even pure polyunsaturated fats have a negative effect on metabolism, sometimes even with their supposedly "healthy" effects. We can mention, for example, the activation of gluconeogenesis, i.e. an increase in blood sugar, or the activation of the release of arachidonic acid from phospholipids and thus an increase in blood clotting, the activation of CB1 cannabinoid receptors, i.e. an increase in appetite, etc. It is therefore not only about the secondary products of autoxidation of these fats, which are clearly toxic.
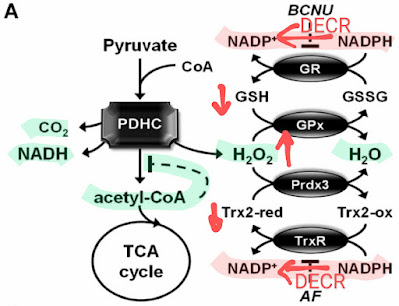 |
| Burning of polyunsaturated fats (DECR) has a similar effect as deactivating the antioxidant chain with BCNU/AF. The pyruvate dehydrogenase complex (PDHC) is the main enzyme enabling the oxidation of carbohydrates. It is mainly deactivated by its end products, NADH and acetyl-CoA molecules. The activity of the antioxidant chain together with scavenging NADH via NNT prevents the deactivation of PDHC. Carnitine does the same thing by taking away acetyl-CoA. Pyr pyruvate, Pyr+Carn pyruvate together with carnitine. Carnitine flushes acetyl-CoA from mitochondria and increases NADH levels. This leads to increased activation of the antioxidant chain. Only after its deactivation (+BCNU/AF) does the actual production of peroxide H2O2 become apparent. |
I will show you my proposal for a fairly simple mechanistic explanation for the increased oxidative stress caused by burning these oils, based on the results of several interesting scientific studies.
Every cell has an excellent antioxidant system hidden in the mitochondria, which uses the NNT enzyme. It is so great that under normal circumstances, all the hydrogen peroxide that is produced is immediately processed into water without fundamentally affecting the cell. The peroxide is simply not visible. It also consumes some energy, so this process "heats". It is controlled by NADH in such a way that, in case of an excess of NADH, the generated peroxide allows the resting consumption of NADH to be increased via NNT. If more intermediate fuel is needed to make ATP, the pathway through the NNT will be suppressed, there will be not enough NADP+ for it. This process serves to dispose excessive intermediate fuel NADH for heat just when not as much chemical energy in the form of ATP is needed. A wonderful system.
Polyunsaturated fats with an even-numbered unsaturated bond, i.e. omega-6 linoleic acid, will "spill sand" into this process, because they use the enzyme 2,4-Dienoyl-CoA reductase (DECR) for burning. This enzyme consumes NADPH and converts it to NADP+ as the only one in breaking down fats, thus supports NNT activity, but disables the antioxidant chain. NNT is normally active only when insufficient consumption of intermediate fuel NADH is accompanied by an increase in H2O2 production. This bond is eliminated by DECR activity, i.e. consumption of NADPH and production of NADP+, i.e. burning of polyunsaturated fats. Resting consumption of NADH increases, heat is produced, but too much acetyl-CoA is also produced. The process ceases to be controlled by NADH but is slowed down by an excess of acetyl-CoA. In the same way, namely through the consumption of NADPH and the production of NADP+, other processes also "pour sand" into this antioxidant system, namely the processing of large amounts of alcohol or sugar. I already wrote about this. This causes an increase of peroxide level in the cell, which is then misinterpreted by the cell as a lack of oxygen for a given amount of fuel, thus activating processes associated with reprogramming the cells to process glucose by fermentation and to create and store fat. This will reduce oxidative stress, but it's not something we want.
 |
| Suppression of resting metabolism by genetic knockout of NNT (6J) leads to obesity and elevated blood glucose levels compared to mice with functional NNT (6N) enzyme. |
Recently, studies have been appearing that show that it is precisely the reduced resting metabolism, reduced resting energy expenditure and lower body temperature that are behind the tendency to obesity. This is fully consistent with the above mechanism.
Next
References:
Dietary Linoleic Acid Elevates Endogenous 2-AG and Anandamide and Induces Obesity
Detailed evaluation of pyruvate dehydrogenase complex inhibition in simulated exercise conditions






Comments
Post a Comment