Fructose turns off nutrient (amino acid) deficiency detection
A deficiency of amino acids (proteins) in the cell activates the enzyme SIRT2. Activation of the enzyme KHK-C, i.e. the mere presence of fructose, which needs this enzyme for its activation, turns off the enzyme SIRT2. Therefore, fructose controls the acetylation of many enzymes and reprograms metabolism. After reading many studies on fructose, I must conclude that sugar could really be behind the epidemic of obesity, diabetes, high blood pressure, etc., although I still think that the long-term effects of fructose are linked to the consumption of plant polyunsaturated oils, their peroxidation into aldehydes, and the initiation of cellular senescence. This is especially supported by the increase in their consumption in the 20th and 21st centuries. We have been consuming fructose in fruit and honey since time immemorial.
 |
| SIRT2, as an indicator of the deficiency of amino acids, deacetylates ACSS2 and suppresses the formation of new fat droplets. |
Diabetics have their artificial sweeteners, which can further worsen metabolism. For non-diabetics, I would probably recommend using glucose (dextrose) instead of sugar. It seems that glucose, given frequently and in small doses, suppresses the negative effects of fructose and returns metabolism to the right track. However, if glucose (or rapidly absorbable carbohydrates) is administered quickly and in large doses, it induces hyperglycemia, activates the processes of fructose formation from glucose (polyol pathway), and activates the enzyme KHK-C in the same way as dietary fructose. The effects of fructose are most pronounced when combined with fats, which is a very common and irreplaceable part of the human diet. Especially animal fats contain a range of vitamins, and additionally, butter or cream contains butyrate, a short-chain fatty acid for nourishing the intestinal epithelium (surface) of the small intestine. Similarly, glucose in small doses may also probably nourish the intestinal epithelium of the small intestine. The activity of gut bacteria also produces acetate as nourishment for the intestinal epithelium, but this occurs in the large intestine. It does not help the small intestine at all.
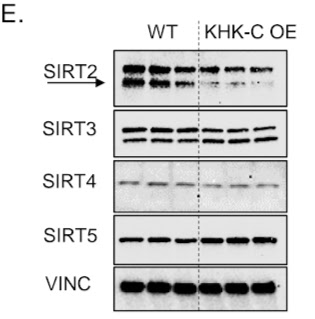 |
| Increasing the amount of the enzyme processing fructose (KHK-C OE) turns off the deacetylase SIRT2 and affects dozens of enzymes by not removing their specific "decoration" with an acetyl group. |
What is deacetylase SIRT2? It is an enzyme that removes the "decoration" of enzymes with an acetyl group. Its activation by amino acid deficiency either increases the activity of some enzymes if the acetyl group interferes with their function (e.g., enzymes for oxidative phosphorylation) or helps remove the enzyme if the release of the acetyl group allows the binding of ubiquitin and leads to their removal (e.g. ACSS2). Therefore, SIRT2 is a signal to conserve amino acids, to avoid wasting them on unnecessary structures, and to avoid wasting energy on fat production. It can also activate enzyme relocation, e.g. deacetylation of FoxO1 moves it to the nucleus, where it suppresses the transcription factor PPAR-gamma. Thus, SIRT2 activation prevents fat formation, which fructose disrupts and promotes fat formation even in nutrient deficiency.
 |
The ACSS2 enzyme processes acetate into acetyl-CoA for acetylation of histones and enzymes and for incorporation into new fats. In the presence of sufficient amino acids (AA+), deactivation of SIRT2 (KD, e.g. with fructose) leads to increased ACSS2 activity and fat storage. Deactivation of SIRT2 in the lack of amino acids (AA-) also slightly increases ACSS2 and fat storage, but statistical significance was not achieved. It seems to me that the effect of nutrient sufficiency alone will increase acetylation and the effect of turning off SIRT2, the effect of fructose on ACSS2 activity increases. Thus, fructose, in combination with sufficient acetate, could induce the observed restart of metabolism in fat cells from pseudohypoxia and senescence towards oxidative phosphorylation. I know that it works in mice, but the mechanism described is pure speculation. |
Another function of SIRT2 is apparently determining the energy source. SIRT2 activation directs metabolism more towards burning fuel using oxygen in mitochondria, while SIRT2 deactivation directs metabolism more towards fermenting glucose into lactate without the need for oxygen. It suppresses the transport of long-chain fatty acids into mitochondria and fat burning by removing CPT1a, see previous post. Oxidative metabolism is more efficient, providing many times more ATP energy with limited fuel sources. Fermentative metabolism is inefficient but fast, allowing rapid disposal of fuel, i.e. glucose from the blood. The body constantly transitions between these states so that it is overall the most advantageous.
 |
| By deactivating SIRT2, fructose directs metabolism away from oxidative phosphorylation towards glycolysis and fermentation. |
Fructose, therefore, masks the signal that tries to conserve amino acids and fuel, doing so regardless of the state of proteins/amino acids. It creates a deficiency of amino acids and cellular energy overall. Instead, it directs all energy and material into fat formation. And it only takes a very small amount to do that.
We have an example in a study on humans, where the effect of fructose on metabolism was examined using sweetened beverages. It is very interesting to see that even when 25% of energy was provided in the form of glucose-sweetened beverages for 10 weeks, it did not essentially cause any major metabolic shocks, unlike fructose beverages, which completely stop energy acquisition from fats and instead induce higher fat formation (negative numbers for fat oxidation). The missing energy is replaced by carbohydrates, which may appear as a favorable reduction in insulin resistance, but it is not so. Researchers state that fructose bypasses protective mechanisms that limits, for example, glucose entry into the cell. This causes a significant fuel surplus in the cell, increases stress, and places great demands on antioxidant protection. I assume that fructose can thus deplete GSH reserves and is unable to ensure its renewal. Glucose can do this, as I have shown in a previous post, even at a concentration of 25 mmol/l; let’s repeat the image in the bottom right.
Thus, fructose erases the resources deficiency signal and directs all energy and material into fat storage. This is typical behavior for storing supplies for winter, which evolution created for surviving long winter periods. However, our civilization can now ensure year-round food availability, and this function of fructose and sugar is troublesome for us, leading to more fat storage, including very unstable and peroxidizing fats. We are creating additional sources of toxins in our bodies as if there were not enough in our environment already.
References:
SIRT2-Mediated ACSS2 K271 Deacetylation Suppresses Lipogenesis Under Nutrient Stress
SIRT2 regulates mitochondrial dynamics and reprogramming via MEK1-ERK-DRP1 and AKT1-DRP1 axes






Comments
Post a Comment