Peroxisomes torn from the chain, how to tame them?
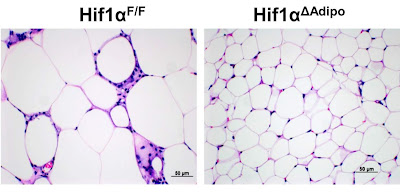
In the previous post, I showed you a mouse study that clearly shows that turning on pseudohypoxia, that is, activating the transcription factor HIF-1α in adipose tissue, triggers obesity and other metabolic problems. I have shown that one of the main triggers is the accumulation of succinic acid (succinate), one of the intermediates of the TCA cycle. If there are any problems with the TCA cycle (obtaining energy through oxidation), succinate builds up and triggers the HIF-1α rescue mechanism, fat cells get bigger and stop working properly.
So the question is how to prevent this? How to reduce the risk of succinate accumulation? How to ensure stable and sufficient breakdown of succinate, i.e. the activity of succinate dehydrogenase (SDH)?
One of the main sources of succinate during fasting, i.e. between meals, are organelles called peroxisomes. They are bound directly to fat triglyceride droplets in the cell and ensure the release of free fatty acids from these triglycerides. At the same time, it can also break down free fatty acids into pairs of carbon atoms of acetyl-CoA using beta oxidation and further convert them into acetate. It can also process omega oxidation products to succinate. In this process, NAD+ molecules are converted to NADH molecules. NADH molecules, as we already know, supply the electron transport chain with energetic electrons, which will create ATP and water molecules as a result.
As the name suggests, peroxisomes produce hydrogen peroxide through their activity. The more they work, the more peroxide. The more peroxide, the more is the decomposition of triglycerides slowed down. This negative feedback mechanism keeps peroxisome activity within appropriate limits. If there is not enough peroxide to slow lipolysis, all the NAD+ molecules are used up, the TCA cycle stops and pseudohypoxia begins. In the event that peroxisomes are the main source of energy for the electron transport chain, the peroxide brake is very important.
Let's review what enzymes break down hydrogen peroxide. It is mainly catalase and glutathione peroxidase. Catalase is directly inside the peroxisome as the main peroxide decomposer. So it controls the balance inside the peroxisome. But a lot of peroxide escapes from the peroxisome into the cytosol, where it is processed by glutathione peroxidase. I already showed a graph here where different free fatty acids activate glutathione peroxidase in different intensity. Omega-6 linoleic acid (C18:2n-6) activates it the most
But this means that if there is an excess of linoleic acid in adipose tissue, hydrogen peroxide will be broken down very quickly, and this will be reflected in the high breakdown of triglycerides into free fatty acids and thus a lot of fuel for peroxisomes. The brake will not work. This will further lead to the induction of NAD+ deficiency and the triggering of pseudohypoxia by HIF-1. Under these conditions, fat-making genes are turned on and the activity of fat-burning genes is reduced. The chain of reactions is quite clear.
But how to prevent it, and how to get out of it? This appears to be prevented by eliminating all sources of omega-6 from the diet. As I explained earlier, it's not that easy, and we usually already have large stores of omega-6 in our adipose tissue. So how to reduce lipolysis, i.e. the breakdown of triglycerides into free fatty acids? This would reduce the activity of peroxisomes, leaving some NAD+ molecules for other processes as well. If you got the idea that nicotinic acid (NA), vitamin B3 Niacin, stops lipolysis in larger doses, then yes, but this is really not the best way, because the mechanism of action causes a so-called rebound effect, things will return to the old ways after a while and skipping supplementation will make things much worse. This is really not the good way. Let's try to do it differently.
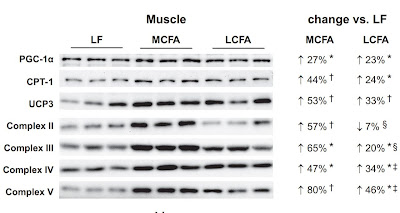
You already know that I promote supplementing with 8 and 10 carbon medium chain oils, ie C8:0 and C10:0 MCT oil. These fats probably reduce lipolysis and beta oxidation in peroxisomes, thus reducing NAD+ consumption, reducing succinate production and allowing metabolism to normalize, at least as a prevention. In addition, they increase the activity of SDH and thus accelerate the breakdown of succinate and delay the risk of triggering pseudohypoxia. On the other hand, coconut oil, which contains mostly lauric acid C12:0, can cause fatty liver. I would be careful there and recommend moderation. You also know that highly diluted hydrogen peroxide therapy should have a similar effect, but take that as information only.
Balanced beta oxidation between mitochondria and peroxisomes is a key to minimizing metabolic problems during fasting (between meals). Unsaturated fatty acids are metabolized more by LCAD in peroxisomes, increase the production of H2O2, increase fat content and deplete the supply of NAD+. Saturated fatty acids are metabolized more by MCAD in mitochondria. We see that fatty liver is induced by increased activation of peroxisomes by CFB. This is due to reduced lipolysis (breakdown of fat droplets) suppressed by increased production of H2O2 inside the peroxisome. If everything is balanced, almost no H2O2 is released from the peroxisomes. However, suppression of omega oxidation (by 4-MP) will greatly increase the level of H2O2 in the cytosol; this will reduce fattening but is likely to induce pseudohypoxia. The cell thus defends itself against excess fuel that cannot be processed.
Important supplement:
Metabolism of unsaturated fatty acids with double bonds at even positions (eg, omega-6 linoleic acid) in peroxisomes is dependent on NADPH produced in the peroxisomal matrix by a mechanism bypassing the TCA cycle. Therefore, linoleic acid apparently affects the metabolism in a fundamental way and creates shortcuts that bypass the regulatory mechanisms that use superoxide and hydrogen peroxide to regulate the supply of fuel to the mitochondrion. This also explains the fact that polyunsaturated conjugated linoleic acid, in contrast to regular linoleic acid, is not harmful and, according to a number of studies, is even beneficial. It has unsaturated bonds only at odd positions.
You can also check out this post about peroxisomes.
References:
Fasting induces hepatic lipid accumulation by stimulating peroxisomal dicarboxylic acid oxidation
Peroxisomal β-oxidation acts as a sensor for intracellular fatty acids and regulates lipolysis
Fatty acid-mediated effects on the glutathione redox cycle in cultured endothelial cells
Accumulation of systemic succinate controls activation of adipose tissue thermogenesis
Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase










Comments
Post a Comment