The epidemic of obesity and metabolic disorders is in full swing, only no one really knows why. If you look at old films or photographs, you will find very few people who are a bit overweight, even in affluent society. People ate what they wanted. Sugar consumption was on the rise, but it manifested itself more in rotten teeth than obesity. But today? Do you think that's been solved? Why, despite constant sports and weight training with a relatively high risk of injury or damage to some part of the body, the obesity curve in the population continues to rise. Where is the cause?
Recently, a theory has emerged that the AhR receptor may be behind this. This receptor switches on when we eat and off after we eat. However, in obese people, this receptor never turns off. It has to do with the poisons in our food, e.g. dioxins reliably switch on this receptor. If you are interested, listen to the YouTube video series created and still being created by Brad Marshall. Maybe we'll look at it in a future post.
I will try to explain why and how cells tend to store fat in a slightly different way. As a pre-programmed process by nature that is normally triggered when insulin levels are high enough. This is because it signals an abundance, or rather an excess, of food. Food is not wasted, so the excess is stored. We will show that this is caused by suppressing the burning of long saturated fats in the mitochondria (M), so that the proportion of fat burning in the peroxisomes (P) is increased. These seem to be the chemical factories for converting unsaturated fats into new saturated ones (Brad Marshall compares them to shredders of all sorts of weird branched polyunsaturated vegetable oils - poisons). Moreover, it is the activity of peroxisomes that inhibits the entry of fats into the mitochondria. Thus, high insulin levels trigger the fat storage factory. That's normal. What's not normal is when fat is stored even when insulin is two orders of magnitude lower, i.e., even with relative food deprivation. Is that even possible? Yes, it is.


Look at the graphs above and compare the P/M ratio for the first graph for an insulin value of -9 (10E-9) i.e. 1 nM and for a value of -7 (1E-7) i.e. 100 nM. The top graph shows the rate of burning palmitic acid, approximately the rate of burning saturated fat. A value of 1 nM is a low level, a value of 100 nM is a high level when eating. We are interested in the ratio, not the absolute magnitude. We see that the P/M ratio is much higher at higher insulin levels. Fat burning in the mitochondria is suppressed, but not at low insulin levels. This is true for the long saturated fats commonly found in traditional food.
However, in the second graph you can see that the same P/M ratio as at 100 nM can be achieved at an insulin level of 1 nM. How is this possible? Just burn omega-6 polyunsaturated arachidonic acid. Because mitochondria don't like unsaturated fats very much, and polyunsaturated fats even less. Peroxisomes, on the other hand, welcome unsaturated fats and burn them preferentially. They also preferentially burn very long saturated fatty acids, but there aren't that many of them.
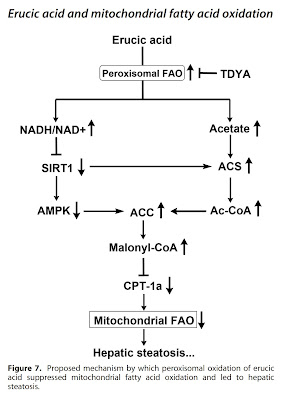
That's the whole problem, an excess of (poly)unsaturated fats leads to fat burning in peroxisomes even at relatively low insulin levels. This leads to fat storage because the peroxisomes dump the raw materials for fat production (acetyl-CoA) into the cell cytosol, block the entry of fats into the mitochondria (via malonyl-CoA), and in addition provide good access to glucose. They let it into the cell by increased production of hydrogen peroxide (H2O2). Glucose is the raw material for the production of glycerol, which binds free fatty acids into triglycerides. These are then stored as fat droplets. In addition, glucose consumed by the body and stored in extra fat can be missed by the brain with all sorts of consequences.
And that's it, this is how PUFAs and oleic acid contained in table oils directly control fat storage. This process is fine and allows survival in nature, where sometimes there is plenty and sometimes there is a lack of food. And when do problems arise? When this situation goes on for too long. Peroxisomes produce quite a lot of hydrogen peroxide, which is neutralized by the increased activity of glutathione peroxidase, which needs enough glutathione to function. This will eventually run out. And then the problems start.
The lack of glutathione, together with high peroxisome activity, will increase H2O2 levels and increase aldehyde production. It also lets glucose into the cell, but it can't go back and it has nowhere to go forward. High levels of acetate from peroxisomes will cause acetylation of enzymes, especially mitochondrial complex I. NADH levels will rise and NAD+ will become deficient. The only pathway for glucose is to turn the lactate dehydrogenase (LDH) reaction toward lactate production. This is released out of the cell, but only after pseudohypoxia is activated (HIF-1α is activated). LDH activity towards lactate production will also suppress peroxisomes, which also convert pyruvate to lactate, compete with LDH and limit each other. This will also reduce H2O2 production, increase free fatty acid levels, further activate glutathione peroxidase and cause insulin resistance. And now we know why genetic deactivation of HIF-1α will prevent the negative effects of a high-fat diet containing polyunsaturated fats. We have already discussed this here. And what do MCT oils do?

Medium-chain fats (MCTs) are not limited by peroxisome activity and can enter mitochondria regardless of malonyl-CoA levels. Thus, they alter the P/M ratio in favour of fat burning in the mitochondria and not towards storage.
We can also show a few graphs from studies in which animal models were targeted to increase (CFB) and decrease (TDYA) peroxisome activity with chemicals while consuming a standard diet (N), a diet enriched with olive oil (HOO), a diet enriched with low-erucic acid (LRO) canola oil, and a diet enriched with high-erucic acid (HRO) rapeseed oil. The canola grown today is bred to contain only a maximum of 2 % erucic acid, so it is considered as safe.
 |
| Comparison of the oxidation of palmitic saturated C16 and erucic unsaturated C22:1 acid in rat liver. |
 |
| Increase of oxidation in peroxisomes, standard diet, olive oil (HOO), canola oil LRO) and erucic acid (HRO). |
 |
| Liver hydrogen peroxide production in peroxisomes, standard diet, olive oil (HOO), canola oil LRO) and erucic acid (HRO), CFB over-activates and TDYA blocks peroxisomes. |
 |
| Liver fat, standard diet, olive oil (HOO), canola oil LRO) and erucic acid (HRO), CFB over-activates and TDYA blocks peroxisomes. |
 |
| Daily weight gain |
 |
| Increased glucose levels after the infusion test (insulin resistance as a direct consequence of increased peroxisome activation). |
 |
| Activation of mitochondrial fat oxidation blocking by malonyl-CoA. |
 |
| Suppression of ketone formation, palmitate entry is blocked, MCT (octanoate) is not blocked |
 |
| Effect of peroxisomes and fat composition on fat oxidation in mitochondria. |
 |
| Hepatic ACC is an indicator of fat formation. |
 |
| Acetyl-CoA is a material for the formation of fats. |
|
|
 |
| Hepatic SIRT1 indicates deacetylation activity, i.e. re-activaton of slowed metabolism. |
 |
| Activation of peroxisomes causes a deficiency of NAD+ and an excess of NADH (fuel for oxidation). |
Little correction:The first picture should look like this
The difference is that the physiological values of insulin are two orders of magnitude lower than in the first picture. Thus, during fasting (-11), more peroxisomes are activated regardless of the type of fat burned. This means that a saving mechanism is activated during fasting, fats and sugars are saved. Things change when insulin becomes elevated, during and after meals (-9). Saturated fats, such as palmitic acid C16:0, suppress the burning of fat in peroxisomes, which will allow undisturbed fat burning in the mitochondria and promote the release of energy by heat or other activity. Unsaturated fats, on the other hand, will keep the peroxisomes running, suppress fat burning in the mitochondria, the body will remain in energy saving mode, and fats will be newly formed and stored.
Moreover, combustion in peroxisomes is mainly limited by the amount of available NAD+ and thus by the availability of oxygen. However, peroxisomes limit the entry of fuel into the TCA cycle, thus limiting CO2 production. In this post, you will learn how important CO2 is for sufficient oxygenation of the tissues. If enough oxygen can be maintained, the NAD+ level is restored and the metabolism is accelerated. If it is deficient, the state of hypoxia is activated and the metabolism is slowed down. This would explain the huge effect that proper nasal breathing, which retains CO2 in the body, has on metabolic rate, especially when burning fat. Unsaturated fats promote hypoxia, which forces us to breathe more deeply or through our mouth and depletes us of CO2. This natural behavior further depletes tissues of oxygen and deepens tissue hypoxia, especially in adipose tissue.




















Comments
Post a Comment