Lactate shuts down glucose oxidation via magnesium ions.
I have discussed here many times that I consider suppressed energy metabolism of cells to be the main problem related to diseases of civilization. They simply for whatever reason, and there may be many of them, operate in energy-saving mode and conserve whatever they can. So they gradually suppress the consumption of saturated fats and glucose and store them as fat. There are then only few options left to awaken the energy mechanisms and increase the metabolic rate.
If we use unsaturated fats to turn on peroxisomes, the entry of saturated fats into the mitochondria will be limited (via malonyl-CoA), carbohydrate burning in the mitochondria will also be limited (via phosphorylation of the PDH complex). Thus, for fuel processing, we are left with only peroxisomes processing unsaturated fats into acetate, and dicarboxylic acids, which ultimately produce succinate. In doing so, pyruvate will be converted to lactate in the peroxisomes. But glucose will compete in this, it will not be oxidized but will also be fermented by the conversion of pyruvate to lactate.
These two processes compete and only depend on the NADH/NAD+ ratio, with excess NADH, glucose fermentation will win out, so even the burning of unsaturated fats in the peroxisomes will eventually be suppressed because it causes excess NADH. In this situation, the cell is either rescued by HIF-1 activation (e.g. if fructose is present) or undergoes apoptosis, i.e. cellular recycling, and is disassembled (without fructose). Some cells should not be rescued, e.g. fat cells. These, if not functioning properly, can damage the whole organism. Shutting down HIF-1α in fat cells will prevent this.
What fuel would be able to bypass this closed doorway to the mitochondria? We already know about MCT oil. The medium and short chain fats are not blocked, this is also true for succinate as a product of the processing of dicarboxylic fatty acids formed in the cell by the omega oxidation of fats.
Or the glucose pathway could be poked (dephosphorylate the PDH complex) with insulin. It probably can be done, e.g. by acute oxidative stress (exercise) or a single dose of more carbohydrate without fructose (sugar), as Brad Marschall suggests. This is probably how Dr. Kempner's rice diet, which I mentioned earlier, works as well.
What is the hallmark of metabolic problems? Increased lactate production. I've mentioned it here before as an intermediate that the body uses when an organ or cell can't process glucose by oxidation down to carbon dioxide, perhaps due to lack of oxygen. In such a case, the cell carries out fermentation. We have also recognized that elevated lactate levels are also an important signal that reprograms the cell's metabolism, opening the lactate transporters MCT4 through the activation of a signal indicating hypoxia (HIF-1).
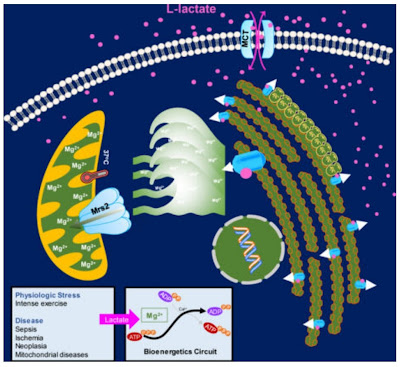 |
| Lactate releases Mg2+ ions that enter the mitochondria via Mrs2 transporters and thus suppress glucose oxidation. |
Recently, studies have emerged that shed light on an important part of this signalling. It appears that lactate directly activates phosphorylation of the PDH complex via magnesium Mg2+ ions. This means that lactate suppresses glucose burning in the mitochondria. This will promote the direction of glucose to further fermentation and higher lactate production, so it is a positive feedback loop (switch). As soon as the cell starts producing lactate, for whatever reason, it switches over and suppresses oxidative phosphorylation, deems it non-functional, and reprograms itself accordingly to a metabolism that it doesn't need oxygen for.
And an important part of this signaling is magnesium released from an organelle called the endoplasmic reticulum. The more magnesium is released when stimulated by lactate, the more of it is taken up by the mitochondria and the more calcium-dependent processes in the mitochondria are inhibited, especially the regulatory enzyme for glucose processing that converts pyruvate to acetyl-CoA, the pyruvate dehydrogenase complex (PDH complex). Here, magnesium and calcium act in opposition to each other, calcium activating dephosphorylation of PDH, magnesium promoting phosphorylation and thus deactivation of the PDH complex.
Foods and poisons that induce lactate production in the cell should be avoided if possible. Among the most problematic poisons are the products of spontaneous oxidation of table seed oils. Another source of poisons is our digestive system, specifically the so-called lipopolysaccharides (LPS), which are formed there by the activity and mainly the breakdown of bacteria. The integrity and health of the intestinal wall determines how much of these poisons enter the blood and thus other organs. Of course, also disease, when dead bacteria activate the production of lactate and thus trigger the body's immune response. Lack of oxygen and poor breathing (hyperventilation) can promote lactate production too.
On the other hand, substances that carry out the so-called chelation of magnesium ions in the cytosol could work against lactate. Also, turning off the transporters for magnesium entry into the mitochondria will completely suppress the slowing of metabolism and the development of obesity in mice fed a high-fat diet. Perhaps this mechanism could also explain the protective effect of azelaic acid in mice on a fatty diet. Succinate or malate could have a similar effect. If the amount of free magnesium is limited by magnesium ions being bound in complexes with dicarboxylic acids, they cannot reach the mitochondria and thus cannot deactivate calcium-dependent processes. Azelaic complexes (aza) with magnesium are probably much more stable than with calcium. We shall see what further research will bring.
References:
Lactate Elicits ER-Mitochondrial Mg2+ Dynamics to Integrate Cellular Metabolism




Comments
Post a Comment