What is mitochondrial uncoupling, and is it good or bad?
If we imagine the mitochondria as a steam power plant with a steam boiler and a generator, then we could compare mitochondrial uncoupling to a safety valve on a steam boiler. If we use a lot of electricity, everything runs as it should. But if we don't need electricity, we have to reduce the energy input to the generator. The pressure inside the steam boiler starts to rise and we have to let some of the steam out so that the boiler doesn't crash. In the mitochondrion, the electrical generator is complex V and the steam boiler is the inner mitochondrial membrane, where electrical voltage is created and maintained with the help of complexes I to IV. The discharge of voltage occurs precisely at complex V, where ATP molecules are formed. Mitochondrial uncoupling proteins (UCP) ensure controlled voltage discharge so that mitochondria are not damaged.
There are two of these proteins/enzymes, UCP1 and UCP2. We will be interested in UCP2, because it is practically in every tissue, in every cell, in every mitochondrion. UCP1 is only active in brown adipose tissue, which serves as our heater, keeping us warm, but there is very little of it in the body. The UCP2 protein can also heat up like UCP1, but much less, and its effect is therefore mainly to protect the mitochondria from overload. If overload is imminent, mitochondria produce more superoxide (O2-) and hydrogen peroxide (H2O2). This activates the formation of the protein UCP2, which then discharges the electrical voltage by allowing some of the protons to pass through the membrane, thus creating an electrical discharge resistor, the shunt. This breaks the link between fuel oxidation and ATP production. For the same amount of ATP, more fuel and more oxygen are needed. Or with the same fuel oxidation, less ATP is produced and the rest is heat energy. UCP enzymes reduce the efficiency of ATP formation processes and thus partially separate these processes from each other, which is why it is called uncoupling.
 |
| UCP1 protein is active only in brown adipose tissue, UCP2 is active throughout the body. But the most important is probably its activity in the pancreas, where it affects the production of insulin. |
The UCP2 protein is activated in a situation where it is necessary to let off steam, i.e. whenever there is too much voltage on the inner mitochondrial membrane. It is arranged in such a way that excess energy electrons from the food are captured on oxygen and form molecules of superoxide O2-, which is immediately converted into hydrogen peroxide H2O2 by the enzyme superoxide dismutase (SOD). A static geomagnetic field is good for this, as I already wrote here. The hydrogen peroxide releases the fatty acids from the near by membrane and UCP2 uses them for its activity and thus lowers the voltage on the membrane. This is most evident in the beta cells of the pancreas, which produce insulin.
Insulin is a very interesting hormone. It controls the whole body, in fact it is a kind of "peroxide level remote communication signal". Beta cells monitor blood glucose levels and produce ATP molecules from glucose and fat in the mitochondria. The more glucose, the more ATP and the more insulin is released out of the beta cell. During this, superoxide and H2O2 are also produced in the mitochondria, this information is transmitted throughout the body by insulin. If too much is produced, the overpressure valve UCP2 is activated and the voltage and the production of ATP and insulin are reduced, but only in the beta cells. Not in adipose tissue. This means that the fat cell will further supply the beta cells with free fatty acids and activate UCP2 in them even more.
It's a defense. If H2O2 production is within normal limits, insulin informs the whole body, telling the cells to increase activity and thus H2O2 production. This transports glucose from the blood to all cells and is processed, usually in the muscles and liver, into glycogen. Hydrogen peroxide is also the main activator of fat storage in adipose tissue. All this is communicated by beta cells to the whole body, and they also receive feedback through the level of free fatty acids (FFA). These at low levels also activate insulin production and stabilize insulin and free fatty acid levels in a controlled manner, the transmitter aligns with the receiver so that H2O2 production is optimal. At higher FFA levels, however, UCP2 will be de-synchronized, will not be activated in adipose tissue, and will be strongly activated in beta cells.
So if there is an increase in the level of free fatty acids (FFA), or if there is an intruder in this insulin information transmission system, the information will be distorted, it will not be transmitted correctly. What intruder am I referring to? Another source of hydrogen peroxide.
We have already seen here that too high production of H2O2 can switch some cells to anaerobic mode, the transcription factor HIF-1α is activated in them and it then activates the NADPH oxidase NOX2 enzyme. This is a large and permanent source of peroxides. Or linoleic acid, elongated to arachidonic acid and placed in the membrane as a phospholipid, can start a chain reaction that activates xanthine oxidase (XO), that's another huge and permanent source of H2O2. There are certainly far more parasitic sources of peroxide.
Another element of the communication system is the liver. It seems that it have none to do in this system, but the opposite is true. It affects the feedback of information using the FFA. On the one hand, the liver collects FFA as triglycerides in VLDL particles, so they do not reach the beta cells. Moreover, it can process FFA into acetate in the peroxisomes, and this, on the other hand, gets into the beta cells and increases insulin production via GPR41/GPR43 receptors and suppresses lipolysis in adipose tissue, so level of long FFA will be decreased. While stimulation via GPR40 receptors is at least partially explored, acetate is not, which is a shame. Acetate appears to be the key to repairing broken insulin communication.
 |
| Increased production of H2O2 leads to the activation of all pathways required for fat storage and obesity (WG). |
 |
| Removing the source of H2O2 (12/15-LOX-- + HFD) prevents obesity. |
On the transmitting side, it is more complicated, for example, a permanently elevated glucose level activates UCP2 so effectively that increased insulin production occurs already at a low glucose level, but no further increase occurs at all, the signal is blocked. It can be increased only by adding the H2O2 signal directly by supplying peroxide. This releases free fatty acids from the local phospholipids of the mitochondrial membrane, and these activate the parallel pathway of insulin release.
This process has intrigued some scientists so much that they propose a procedure to increase insulin production by deactivating the protection of UCP2. Well, I don't know, it seems somewhat risky to me, although insulin production is normalized, but in the long term, probably at the cost of destroying mitochondria. This in turn reduces insulin production, possibly irreversibly. But it works in mice on a high fat diet, see the picture. The mice neither have inflammation nor are they obese, interesting.
 |
| Genetic inactivation of UCP2 (UCP2 KO) prevents obesity in high-fat diet (HFD) mice. |
The process of controlling insulin release from beta cells is really dependent on many parameters. It has at least three components that work side by side - GSIS, RSIS and FASIS + GPR (stimulation by glucose, H2O2 and fat + fat GPR receptors). Interestingly, turning off UCP2 in a high fat environment has the opposite effect to that of glucose stimulation alone. With glucose, turning off UCP2 leads to an increase in insulin secretion, but in the presence of palmitic acid, turning off UCP2 leads to a decrease in insulin production. This could also account for the effectiveness of turning off UCP2 against obesity in mice. Who knows?
I don't know if a definite conclusion can be drawn from these studies. I would hold that the insulin information system of the whole body is only functional if it is not disturbed by false sources of H2O2, this brings me back to the fact that we need to prevent the switching of HIF-1α in adipose tissue and we also need to prevent the activation of xanthine oxidase (XO) by products of metabolism of linoleic acid. So try to keep your omega-6 intake to a minimum and breathe well, slowly, to retain as much CO2 as possible in the body and ensure good oxygen delivery to the tissues. Oxygen constantly circulating in the blood is useless to the tissue.
References:
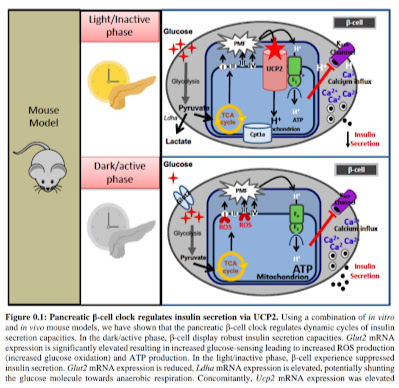
Investigating the metabolic and molecular regulators of diurnal insulin secretion
A role for uncoupling protein‐2 as a regulator of mitochondrial hydrogen peroxide generation
UCP2 KO mice exhibit ameliorated obesity and inflammation induced by high-fat diet feeding





Comments
Post a Comment